Chapter 18: Life On Earth
Chapter 1
How Science Works
- The Scientific Method
- Evidence
- Measurements
- Units and the Metric System
- Measurement Errors
- Estimation
- Dimensions
- Mass, Length, and Time
- Observations and Uncertainty
- Precision and Significant Figures
- Errors and Statistics
- Scientific Notation
- Ways of Representing Data
- Logic
- Mathematics
- Geometry
- Algebra
- Logarithms
- Testing a Hypothesis
- Case Study of Life on Mars
- Theories
- Systems of Knowledge
- The Culture of Science
- Computer Simulations
- Modern Scientific Research
- The Scope of Astronomy
- Astronomy as a Science
- A Scale Model of Space
- A Scale Model of Time
- Questions
Chapter 2
Early Astronomy
- The Night Sky
- Motions in the Sky
- Navigation
- Constellations and Seasons
- Cause of the Seasons
- The Magnitude System
- Angular Size and Linear Size
- Phases of the Moon
- Eclipses
- Auroras
- Dividing Time
- Solar and Lunar Calendars
- History of Astronomy
- Stonehenge
- Ancient Observatories
- Counting and Measurement
- Astrology
- Greek Astronomy
- Aristotle and Geocentric Cosmology
- Aristarchus and Heliocentric Cosmology
- The Dark Ages
- Arab Astronomy
- Indian Astronomy
- Chinese Astronomy
- Mayan Astronomy
- Questions
Chapter 3
The Copernican Revolution
- Ptolemy and the Geocentric Model
- The Renaissance
- Copernicus and the Heliocentric Model
- Tycho Brahe
- Johannes Kepler
- Elliptical Orbits
- Kepler's Laws
- Galileo Galilei
- The Trial of Galileo
- Isaac Newton
- Newton's Law of Gravity
- The Plurality of Worlds
- The Birth of Modern Science
- Layout of the Solar System
- Scale of the Solar System
- The Idea of Space Exploration
- Orbits
- History of Space Exploration
- Moon Landings
- International Space Station
- Manned versus Robotic Missions
- Commercial Space Flight
- Future of Space Exploration
- Living in Space
- Moon, Mars, and Beyond
- Societies in Space
- Questions
Chapter 4
Matter and Energy in the Universe
- Matter and Energy
- Rutherford and Atomic Structure
- Early Greek Physics
- Dalton and Atoms
- The Periodic Table
- Structure of the Atom
- Energy
- Heat and Temperature
- Potential and Kinetic Energy
- Conservation of Energy
- Velocity of Gas Particles
- States of Matter
- Thermodynamics
- Entropy
- Laws of Thermodynamics
- Heat Transfer
- Thermal Radiation
- Wien's Law
- Radiation from Planets and Stars
- Internal Heat in Planets and Stars
- Periodic Processes
- Random Processes
- Questions
Chapter 5
The Earth-Moon System
- Earth and Moon
- Early Estimates of Earth's Age
- How the Earth Cooled
- Ages Using Radioactivity
- Radioactive Half-Life
- Ages of the Earth and Moon
- Geological Activity
- Internal Structure of the Earth and Moon
- Basic Rock Types
- Layers of the Earth and Moon
- Origin of Water on Earth
- The Evolving Earth
- Plate Tectonics
- Volcanoes
- Geological Processes
- Impact Craters
- The Geological Timescale
- Mass Extinctions
- Evolution and the Cosmic Environment
- Earth's Atmosphere and Oceans
- Weather Circulation
- Environmental Change on Earth
- The Earth-Moon System
- Geological History of the Moon
- Tidal Forces
- Effects of Tidal Forces
- Historical Studies of the Moon
- Lunar Surface
- Ice on the Moon
- Origin of the Moon
- Humans on the Moon
- Questions
Chapter 6
The Terrestrial Planets
- Studying Other Planets
- The Planets
- The Terrestrial Planets
- Mercury
- Mercury's Orbit
- Mercury's Surface
- Venus
- Volcanism on Venus
- Venus and the Greenhouse Effect
- Tectonics on Venus
- Exploring Venus
- Mars in Myth and Legend
- Early Studies of Mars
- Mars Close-Up
- Modern Views of Mars
- Missions to Mars
- Geology of Mars
- Water on Mars
- Polar Caps of Mars
- Climate Change on Mars
- Terraforming Mars
- Life on Mars
- The Moons of Mars
- Martian Meteorites
- Comparative Planetology
- Incidence of Craters
- Counting Craters
- Counting Statistics
- Internal Heat and Geological Activity
- Magnetic Fields of the Terrestrial Planets
- Mountains and Rifts
- Radar Studies of Planetary Surfaces
- Laser Ranging and Altimetry
- Gravity and Atmospheres
- Normal Atmospheric Composition
- The Significance of Oxygen
- Questions
Chapter 7
The Giant Planets and Their Moons
- The Gas Giant Planets
- Atmospheres of the Gas Giant Planets
- Clouds and Weather on Gas Giant Planets
- Internal Structure of the Gas Giant Planets
- Thermal Radiation from Gas Giant Planets
- Life on Gas Giant Planets?
- Why Giant Planets are Giant
- Gas Laws
- Ring Systems of the Giant Planets
- Structure Within Ring Systems
- The Origin of Ring Particles
- The Roche Limit
- Resonance and Harmonics
- Tidal Forces in the Solar System
- Moons of Gas Giant Planets
- Geology of Large Moons
- The Voyager Missions
- Jupiter
- Jupiter's Galilean Moons
- Jupiter's Ganymede
- Jupiter's Europa
- Jupiter's Callisto
- Jupiter's Io
- Volcanoes on Io
- Saturn
- Cassini Mission to Saturn
- Saturn's Titan
- Saturn's Enceladus
- Discovery of Uranus and Neptune
- Uranus
- Uranus' Miranda
- Neptune
- Neptune's Triton
- Pluto
- The Discovery of Pluto
- Pluto as a Dwarf Planet
- Dwarf Planets
- Questions
Chapter 8
Interplanetary Bodies
- Interplanetary Bodies
- Comets
- Early Observations of Comets
- Structure of the Comet Nucleus
- Comet Chemistry
- Oort Cloud and Kuiper Belt
- Kuiper Belt
- Comet Orbits
- Life Story of Comets
- The Largest Kuiper Belt Objects
- Meteors and Meteor Showers
- Gravitational Perturbations
- Asteroids
- Surveys for Earth Crossing Asteroids
- Asteroid Shapes
- Composition of Asteroids
- Introduction to Meteorites
- Origin of Meteorites
- Types of Meteorites
- The Tunguska Event
- The Threat from Space
- Probability and Impacts
- Impact on Jupiter
- Interplanetary Opportunity
- Questions
Chapter 9
Planet Formation and Exoplanets
- Formation of the Solar System
- Early History of the Solar System
- Conservation of Angular Momentum
- Angular Momentum in a Collapsing Cloud
- Helmholtz Contraction
- Safronov and Planet Formation
- Collapse of the Solar Nebula
- Why the Solar System Collapsed
- From Planetesimals to Planets
- Accretion and Solar System Bodies
- Differentiation
- Planetary Magnetic Fields
- The Origin of Satellites
- Solar System Debris and Formation
- Gradual Evolution and a Few Catastrophies
- Chaos and Determinism
- Extrasolar Planets
- Discoveries of Exoplanets
- Doppler Detection of Exoplanets
- Transit Detection of Exoplanets
- The Kepler Mission
- Direct Detection of Exoplanets
- Properties of Exoplanets
- Implications of Exoplanet Surveys
- Future Detection of Exoplanets
- Questions
Chapter 10
Detecting Radiation from Space
- Observing the Universe
- Radiation and the Universe
- The Nature of Light
- The Electromagnetic Spectrum
- Properties of Waves
- Waves and Particles
- How Radiation Travels
- Properties of Electromagnetic Radiation
- The Doppler Effect
- Invisible Radiation
- Thermal Spectra
- The Quantum Theory
- The Uncertainty Principle
- Spectral Lines
- Emission Lines and Bands
- Absorption and Emission Spectra
- Kirchoff's Laws
- Astronomical Detection of Radiation
- The Telescope
- Optical Telescopes
- Optical Detectors
- Adaptive Optics
- Image Processing
- Digital Information
- Radio Telescopes
- Telescopes in Space
- Hubble Space Telescope
- Interferometry
- Collecting Area and Resolution
- Frontier Observatories
- Questions
Chapter 11
Our Sun: The Nearest Star
- The Sun
- The Nearest Star
- Properties of the Sun
- Kelvin and the Sun's Age
- The Sun's Composition
- Energy From Atomic Nuclei
- Mass-Energy Conversion
- Examples of Mass-Energy Conversion
- Energy From Nuclear Fission
- Energy From Nuclear Fusion
- Nuclear Reactions in the Sun
- The Sun's Interior
- Energy Flow in the Sun
- Collisions and Opacity
- Solar Neutrinos
- Solar Oscillations
- The Sun's Atmosphere
- Solar Chromosphere and Corona
- Sunspots
- The Solar Cycle
- The Solar Wind
- Effects of the Sun on the Earth
- Cosmic Energy Sources
- Questions
Chapter 12
Properties of Stars
- Stars
- Star Names
- Star Properties
- The Distance to Stars
- Apparent Brightness
- Absolute Brightness
- Measuring Star Distances
- Stellar Parallax
- Spectra of Stars
- Spectral Classification
- Temperature and Spectral Class
- Stellar Composition
- Stellar Motion
- Stellar Luminosity
- The Size of Stars
- Stefan-Boltzmann Law
- Stellar Mass
- Hydrostatic Equilibrium
- Stellar Classification
- The Hertzsprung-Russell Diagram
- Volume and Brightness Selected Samples
- Stars of Different Sizes
- Understanding the Main Sequence
- Stellar Structure
- Stellar Evolution
- Questions
Chapter 13
Star Birth and Death
- Star Birth and Death
- Understanding Star Birth and Death
- Cosmic Abundance of Elements
- Star Formation
- Molecular Clouds
- Young Stars
- T Tauri Stars
- Mass Limits for Stars
- Brown Dwarfs
- Young Star Clusters
- Cauldron of the Elements
- Main Sequence Stars
- Nuclear Reactions in Main Sequence Stars
- Main Sequence Lifetimes
- Evolved Stars
- Cycles of Star Life and Death
- The Creation of Heavy Elements
- Red Giants
- Horizontal Branch and Asymptotic Giant Branch Stars
- Variable Stars
- Magnetic Stars
- Stellar Mass Loss
- White Dwarfs
- Supernovae
- Seeing the Death of a Star
- Supernova 1987A
- Neutron Stars and Pulsars
- Special Theory of Relativity
- General Theory of Relativity
- Black Holes
- Properties of Black Holes
- Questions
Chapter 14
The Milky Way
- The Distribution of Stars in Space
- Stellar Companions
- Binary Star Systems
- Binary and Multiple Stars
- Mass Transfer in Binaries
- Binaries and Stellar Mass
- Nova and Supernova
- Exotic Binary Systems
- Gamma Ray Bursts
- How Multiple Stars Form
- Environments of Stars
- The Interstellar Medium
- Effects of Interstellar Material on Starlight
- Structure of the Interstellar Medium
- Dust Extinction and Reddening
- Groups of Stars
- Open Star Clusters
- Globular Star Clusters
- Distances to Groups of Stars
- Ages of Groups of Stars
- Layout of the Milky Way
- William Herschel
- Isotropy and Anisotropy
- Mapping the Milky Way
- Questions
Chapter 15
Galaxies
- The Milky Way Galaxy
- Mapping the Galaxy Disk
- Spiral Structure in Galaxies
- Mass of the Milky Way
- Dark Matter in the Milky Way
- Galaxy Mass
- The Galactic Center
- Black Hole in the Galactic Center
- Stellar Populations
- Formation of the Milky Way
- Galaxies
- The Shapley-Curtis Debate
- Edwin Hubble
- Distances to Galaxies
- Classifying Galaxies
- Spiral Galaxies
- Elliptical Galaxies
- Lenticular Galaxies
- Dwarf and Irregular Galaxies
- Overview of Galaxy Structures
- The Local Group
- Light Travel Time
- Galaxy Size and Luminosity
- Mass to Light Ratios
- Dark Matter in Galaxies
- Gravity of Many Bodies
- Galaxy Evolution
- Galaxy Interactions
- Galaxy Formation
- Questions
Chapter 16
The Expanding Universe
- Galaxy Redshifts
- The Expanding Universe
- Cosmological Redshifts
- The Hubble Relation
- Relating Redshift and Distance
- Galaxy Distance Indicators
- Size and Age of the Universe
- The Hubble Constant
- Large Scale Structure
- Galaxy Clustering
- Clusters of Galaxies
- Overview of Large Scale Structure
- Dark Matter on the Largest Scales
- The Most Distant Galaxies
- Black Holes in Nearby Galaxies
- Active Galaxies
- Radio Galaxies
- The Discovery of Quasars
- Quasars
- Types of Gravitational Lensing
- Properties of Quasars
- The Quasar Power Source
- Quasars as Probes of the Universe
- Star Formation History of the Universe
- Expansion History of the Universe
- Questions
Chapter 17
Cosmology
- Cosmology
- Early Cosmologies
- Relativity and Cosmology
- The Big Bang Model
- The Cosmological Principle
- Universal Expansion
- Cosmic Nucleosynthesis
- Cosmic Microwave Background Radiation
- Discovery of the Microwave Background Radiation
- Measuring Space Curvature
- Cosmic Evolution
- Evolution of Structure
- Mean Cosmic Density
- Critical Density
- Dark Matter and Dark Energy
- Age of the Universe
- Precision Cosmology
- The Future of the Contents of the Universe
- Fate of the Universe
- Alternatives to the Big Bang Model
- Space-Time
- Particles and Radiation
- The Very Early Universe
- Mass and Energy in the Early Universe
- Matter and Antimatter
- The Forces of Nature
- Fine-Tuning in Cosmology
- The Anthropic Principle in Cosmology
- String Theory and Cosmology
- The Multiverse
- The Limits of Knowledge
- Questions
Chapter 19
Life in the Universe
- Life in the Universe
- Astrobiology
- Life Beyond Earth
- Sites for Life
- Complex Molecules in Space
- Life in the Solar System
- Lowell and Canals on Mars
- Implications of Life on Mars
- Extreme Environments in the Solar System
- Rare Earth Hypothesis
- Are We Alone?
- Unidentified Flying Objects or UFOs
- The Search for Extraterrestrial Intelligence
- The Drake Equation
- The History of SETI
- Recent SETI Projects
- Recognizing a Message
- The Best Way to Communicate
- The Fermi Question
- The Anthropic Principle
- Where Are They?
Importance of Water for Life
Just as it is a difficult task to concisely define what constitutes life on Earth, it is equally difficult to construct a list of environmental requirements that represents the bare essentials for life. In general, astrobiologists are in agreement that life needs a liquid medium for transporting the molecules that are necessary for its existence. For life on Earth, this liquid medium is water. Many other liquids have been proposed as potential substitutes for water, such as ammonia (NH3), methane (CH4) or ethane (C2H6). However, as we will see, water is a versatile and somewhat unique molecule that is extremely compatible with life on Earth, and probably on other planets. Many of the Earth-like exoplanets that have been found are likely to have environments with water.
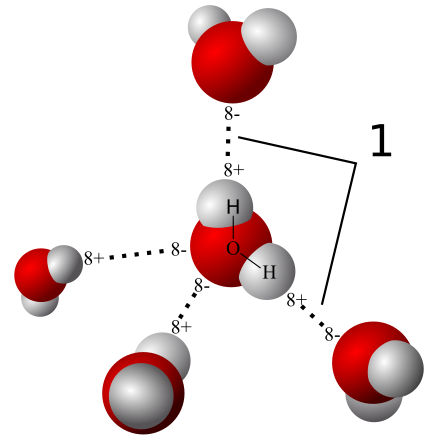
As we begin our discussion of the importance of water for life, it is important to recognize that water is possibly the most abundant liquid in the universe. But does that necessarily make it the best candidate for life? What are the properties of water that make it well suited? Let's begin by examining a single molecule of water. Water is composed of two hydrogen atoms and a single oxygen atom; there are a total of two chemical bonds in the molecule. Each of the hydrogen atoms is bound to the oxygen by a covalent bond — a bond formed by the sharing of one or more electrons between two atoms. In the case of the oxygen-hydrogen covalent bond, the electrons are not shared equally between the two atoms. In fact, oxygen is a strongly electro-negative atom and can be thought of as a bit of an "electron hog". The oxygen will tug on electrons so that there will be a higher density of electrons around the oxygen as compared to the hydrogen. As a result, if you were to examine just one of the covalent bonds in a molecule of water, you would notice that the oxygen atom would be slightly negative and the hydrogen atom would be slightly positive; we would say that the covalent bond is polar. This may not seem important just yet, but the interactions of hydrogen and oxygen atoms between water molecules are the key to understanding the uniqueness of water.
In a water molecule, the oxygen atom can be described as having a net negative charge and the hydrogen atom as having a net positive charge. When two objects with opposite charges are very close to one another, they will experience an attractive force between them, much like when two magnets "stick" together. On the flip side, two objects with similar charges will experience a repulsive force between them. You can probably imagine that the hydrogen atoms of one water molecule then might be attracted to the oxygen atom of another water molecule. The attraction between these two atoms of different molecules is an example of hydrogen bonding. Hydrogen bonding isn't as strong as bonds where electrons are shared or exchanged and can only happen when the molecules are fairly close together. Nevertheless, it is water's propensity to engage in hydrogen bonding that gives it some of its unique characteristics. Hydrogen bonding is responsible for the fact that ice in liquid water floats, that water remains liquid over a large range of temperatures, that water has a high specific heat, and that strands of DNA stay "zipped" together.
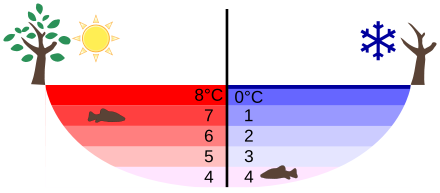
Ever notice that a pond or lake during a cold winter never freezes solid? Ever wonder why? Well, you can blame it all on our friend the hydrogen bond. As liquid water gets colder and colder, the number of hydrogen bonds between water molecules gradually increases. When water freezes and becomes ice, the hydrogen bonds will hold the water molecules in such a way that they form a crystal lattice. In this crystal lattice, the water molecules are actually more spread out than when they are in a liquid state, which means they take up more volume. The actual mass of water molecules does not change. If we increase the volume, yet maintain the mass, we decrease the density of a substance. We can determine this fairly easily from the simple mathematical equation of d = m/V, where d represents density, m represents mass, and V represents volume. Therefore, ice is "less" dense than water, making it float! Let's think about that pond in the cold winter. As it starts to freeze, all of the ice floats to the top. Eventually, the pond might freeze over completely, sealing in a liquid oasis underneath where life can survive the winter. This characteristic of water — becoming less dense when it freezes and therefore making it float — allows aquatic environments to survive from year to year. Can you imagine what would happen to aquatic life if the pond froze solid every winter?
Hydrogen bonding is also responsible for two important properties of water: its ability to remain a liquid over a large temperature range and its high specific heat. Why are these two properties important for life? On Earth, a large percentage of living organisms is comprised of water. Let's imagine that instead of remaining liquid over a temperature range of 100 degrees Celsius, water was only liquid over a range of 20 degrees Celsius. Over the course of a day, temperatures on Earth can vary by greater than 20 degrees. For example, on an extreme day in Arizona, temperatures can go from around 20 degrees Celsius to 45 degrees Celsius. If instead of remaining liquid over that temperature range, water changed from a liquid to a gas and began to boil; the effects could be devastating for life. Imagine, for instance, what would happen to the cells in your body, consisting mostly of water! Or consider the opposite case of a temperature change resulting in water freezing. If the liquid that life relies on were constantly frozen, how would life carry out the necessary chemical reactions? Luckily, water is liquid over a large range of temperatures — 100 degrees Celsius. This range is large enough to ensure that the water in cells will neither freeze nor boil off and therefore make it difficult for life to exist.
Equally important to a large temperature range is the high specific heat of water. Specific heat is just a fancy way of describing the amount of energy that is required to raise the temperature of a substance. In the case of water, it is the amount of energy to raise the temperature of one gram of water by one degree Celsius and is equal to 4.186 Joules (J). Every material has a unique specific heat that depends on its own chemical composition. Water has a particularly high specific heat when compared to other liquids for life. For example, the specific heat of ammonia (NH3) is 0.470 J/°C. If our oceans were made of ammonia instead of water, it would take significantly less energy to change the temperature of the ocean. This means that over the course of a normal year, there would be global temperature changes that could result in widespread flooding and glaciation on an annual basis! The specific heat of water helps Earth maintain its fairly stable climate.
On a more elemental level, hydrogen bonding plays a vital role in the ability for life to reproduce and evolve. The DNA in our cells is double-stranded; there are two strings of nucleotides that are joined primarily by hydrogen bonds between the strands. We mentioned earlier that hydrogen bonds, when considered as individual bonds, are very weak. However, when we consider hundreds of hydrogen bonds together, a relatively strong and stable structure can result. Nucleotides from one strand make two or three hydrogen bonds with complementary nucleotides of the other strand, depending on the nucleotide pair. The two strands together make up the helical DNA molecules that transfer genetic information from one generation to the next. Without these molecules, life would have a very difficult time perpetuating itself on the planet.
So far, we have examined the nature of hydrogen bonding and how it contributes to the importance of water for life. Water has other characteristics that also make it important to life on Earth. First, water acts as an excellent solvent, dissolving a wide variety of materials. As a solvent, water assists in transporting molecules within the cell. In addition, water is what causes proteins to take on their unique three-dimensional shapes, allowing them to catalyze very specific chemical reactions within cells. Finally, water can act as a shield, if enough of it is present. For example, during the history of early Earth, there was not a protective layer in the atmosphere that blocked harmful ultraviolet (UV) radiation. Some astrobiologists contend that the first forms of life arose in the ocean, where depths of water could absorb the UV and protect budding life forms.
Although astrobiologists are not limiting the existence of life to liquid water, due to the versatility of water it is likely that life on other planets might also utilize water as its "life liquid". Consequently, many of the exploratory missions inside and outside of our Solar System focus on the discovery of past or present water on planetary bodies as an indicator of life.

